The Freedom of Knowledge, The Power of Thought ©
Current News | Introduction | Colloidal Silver | Chemtrails | Sylphs | Emerging Diseases | Forbidden Cures | Ozone | Immunity Boosting | Nutrition | Tone Gen
From Ken Adachi, Editor Facebook Censorship
A little background first to help you understand the significance of this information: I first came to realize how important Vit. C is to the proper functioning of all our bodily systems when I read Dr Linus Pauling's book, Vitamin C and the Common Cold in the 1970s. The only thing we were taught in school about Vit. C is that you needed it to prevent scurvy, but that was about it. So Dr. Pauling's book created a lot of curiosity from both eager readers (such as myself) and Medical Establishment debunkers who, as usual, were anxious to pooh pooh Pauling's discoveries about Vit. C's curative capabilities. The topic was brand new at the time and it was fascinating to discover that something as ordinary as Vit. C, just one of many vitamins we were told that we get plenty of from our diet, could have such a profound healing and restorative effect upon the body if taken in large enough doses. After his first book on the common cold, Pauling went on to write other books about the nearly miraculous ability of Vit. C to knock down cancer and other disease conditions - if delivered in sufficiently large amounts to achieve the curative effect. Large amounts of Vit. C on a cellular level could kill cancer cells by producing enough hydrogen peroxide to destroy the cancer cells (healthy cells contain an enzyme called catalase which breaks down hydrogen peroxide, but cancer cells don't produce catalase, so they are vulnerable to the oxidation of hydrogen peroxide).
Prior to viewing this video, the only way I knew how to deliver really large amounts of Vit. C into the bloodstream was to have an intravenous (IV) infusion of Vit C which meant going to a physician's office and paying for an IV drip. And for those who knew about it and could afford it, many undoubtedly benefited from that treatment and could often see amazing results after one or more Vit. C drips, depending on what the problem was. Another idea being promoted today is the use of the Liposomal form of Vit. C, but as this video demonstrates, the Liposomal form doesn't deliver any more Vit. C into the blood than does ordinary Ascorbic Acid; so why spend the money? On the other hand, if you took Vit. C orally using ascorbate or ascorbic acid or Liposomal Vit. C, you could archive good results, but you could only absorb about 10% into your bloodstream of what you ingested . And even then, there's a saturation mechanism that kicks in that creates an upper maximum limit of about 500 mg. of Vit. C in your bloodstream, no matter how many grams of ascorbate or ascorbic acid you consumed. DHAA The GLUT transport molecule is plentiful in the gut because it transports glucose and it requires no extra energy to do its job, so it transports DHAA into the blood at a faster rate and at a substantially higher amount than can the less plentiful SVCT molecule carry ascorbic acid through the gut and into the blood (or into cells). The SVCT molecule is less efficient because as an active transporter, it requires energy, and it's saturable which limits the total amount of ascorbic acid which can be transported into the cell.
(The dashed black line and the solid black line on this chart represent test results measured by other researchers who used 5 grams of AA and Liposomal Vit. C respectively.) As you can see from the chart, when Doug drank the green smoothie containing 5 grams of DHAA, his blood level of Vit. C (solid red) rose to 472 mg. (indicated as uM - micro mols - on the chart ) at the 60 minute mark, which is about double the amount which ascorbic acid, ascorbate, or the Liposomal Vit. C could produce in the blood. He also mentions that this is the HIGHEST level of Vit. C blood plasma ever recorded based on 5 grams of Vit. C intake. While Doug consumed 5 grams of DHAA from the freshly made green smoothie to match the 5 gram test amounts of AA and Liposomal Vit. C used by other researchers, he says that he could have made the smoothie double or even triple strength from what he made on the video. By extension, if he had consumed 10 grams or even 15 grams of DHAA (by drinking the entire 4 cup smoothie), could he have obtained 944 mg. or 1,416 mg. respectively of Vit. C levels in his blood plasma? It would be interesting to find out. If drinking 5,000 mg (= 5 grams) of DHAA produces 472 mg. of blood plasma Vit. C after one hour, then a single ice cube of green smoothie containing 500 mg. of DHAA would produce 47.2 mg. of blood plasma Vit. C after one hour. Ten green smoothie ice cubes would equal 5 grams of DHAA. Twenty green smoothie ice cubes = 10 grams of DHAA. And thirty green smoothie ice cubes = 15 grams of DHAA. The possibility now exists to obtain nearly as high a dose of blood plasma Vit. C from oral dosing with DHAA as one might obtain with an Vit. C IV drip. This could open up huge mega-dosing Vit. C therapy possibilities for people with very serious health conditions like cancer. Details on how to properly make the green smoothie is explained very well in the video below. Pay close attention to the details if you want to do this correctly and achieve the desired results. I've added some additional notes below on making the Starch-Iodine reagent, as well as notes on the Ascorbic acid oxidase enzyme found in zucchini which allows the conversion of ascorbic acid into DHAA. View the video a few times to get all the details straight on making this smoothie correctly. The only way to keep the DHAA viable at 90% potency for 30 days is to freeze it. At room temperature, the green smoothie will lose 25% of its DHAA potency after 12 hours, so freezing it is a must for 30 day storage. Ascorbic acid oxidase found in Zucchini Ascorbic acid oxidase (AAO) is a common enzyme found in many plants, but zucchini has the highest concentration of this enzyme which acts as a catalyst (utilizing oxygen from ambient air) to change ascorbic acid into dehydroascorbic acid. You have to pay attention to his instructions regarding an optimum pH of 6 as well as the the optimal temperature of the smoothie solution at 40 degrees Centigrade to get the very best catalytic results. Starch-Iodine Reagent used to verify Ascorbic Acid conversion into DHAA The Vit. C absorbed into your blood above the normal plasma level would last about 4 hours (unless you took bioflavinoids, which could double the blood elevation time to about 8 hours). Our blood plasma usually contains about 100 mg of Vit. C (shown as 100uM - micro mols - on the chart). Below the transcript, I've added the links to many journal reports and articles which Doug has posted at his web site. Well worth reading to learn more about DHAA. Ken Adachi © Copyright 2017 Educate-Yourself.org All Rights Reserved. Doug Kitt's video Aido file of this video: English Transcript Hello, I'm Doug Kitt with ReCverin company of Salt Lake City, Utah. Today I'm going to
show how you can make your own dehydroascorbic acid, a form of oxidized vitamin C, for supplementing your diet. And as the title implies, we're going to be making a vegetable puree that is often referred to as a "green smoothie." But what you'll learn is that, in scientific terms, we're going to conduct a natural, biochemical synthesis reaction, and we'll utilize a sensitive
redox indicator to monitor the progress of this reaction. In short, I'm going to teach you how to be a successful biochemist! And amazingly, we're going to do this in the kitchen at home with a blender, plain old zucchini squash, common vitamin C, and other materials that you probably already have or that you can easily and economically obtain.
But maybe you're asking yourself, "Why would I want to make a dehydroascorbic acid dietary supplement? What is this stuff, what's so special about it, and why would I want to use it instead of my current vitamin C supplement?" Well, these are all things we are going to
cover in this video. But one reason is to raise the level of vitamin C in your bloodstream higher than can be achieved by eating regular old vitamin C tablets. Liposomal vitamin C claims to accomplish this; unfortunately, it doesn't appear to. But before I get to discussing why you will want to use dehydroascorbic acid instead of liposomal vitamin C, I think
we should take a few minutes to discuss the natural ways that vitamin C is absorbed. After that we'll examine some scientific literature to see just what real evidence there is to
support using liposomal vitamin C to increase vitamin C absorption, and why you should consider switching to something better. Common vitamin C. Each of the tablets in this jar contains 1000 milligrams, or one gram of l-ascorbic acid. This is the most common supplemental form of vitamin C you will find, but you should be aware that there are others. In fact, if we take a look at the Wikipedia
Vitamin C webpage, this is what we find: "Vitamin C refers to a number of vitamers that have vitamin C activity in animals, including ascorbic acid and its salts, and some oxidized forms of the molecule like dehydroascorbic acid." And this time, no hocus pocus. This time, let's talk about a well-known, scientifically-valid
mechanism that has been proven in many scientific studies. Let's talk about an alternative pathway
for absorbing vitamin C, one that your body already has built-in. DHAA is absorbed by the cells in your digestive tract using a different transport protein than AA, a transporter called GLUT. Let's look again at the teaching materials from the Kansas City University nutrition class.
The name GLUT, or G L U T, is derived from the words "glucose transporter," and comes from the fact that these transporters were first discovered as proteins for transporting glucose, or sugar, into the cells. Interestingly, these transport proteins are multi-functional, and it was later discovered that they are also the transporters for DHAA. Well, since glucose is the most common sugar in the diet, your intestinal cells have a lot of these GLUT transporters. That means your intestinal cells have a great capacity to absorb DHAA. And on top of that, GLUT transporters are a type known as "passive transporters." Passive
transporters don't require any extra energy for absorption and therefore move DHAA into the cells much faster than SVCT transports AA. DHAA is converted into AA once it gets inside the cell. There is an enzyme called Ascorbic Acid Oxidase or AAO. It's common in many plants, but zucchini squash has a higher concentration of it than any other known source. AAO catalyzes this reaction, the oxidation of ascorbic acid to dehydroascorbic acid. Now all we're going to do, basically, is puree zucchini in the blender and add AA to it. The enzyme in the zucchini will catalyze the conversion of AA into DHAA using oxygen from the air. But we need to be careful to control certain conditions of the reaction to make sure it works, including the pH or acidity, the concentrations of the reactants, and the temperature Take a look at this graph. The AAO enzyme has a pH range that is optimal for its activity. We need to keep our puree within this optimal pH range, and very importantly we don't want to exceed certain pH levels. If we make the solution too acidic, it might kill the activity of the enzyme completely and the reaction won't work. On the other hand, even though
the enzyme is active at high pH, DHAA is unstable at high pH and breaks down quite rapidly
So we need to balance the pH carefully. The enzyme also has an optimal temperature range. I'll explain why this is important and how we control it in just a few minutes. So at this stage we're trying to minimize the actual amount of mixing time while still
assuring that it gets fresh air periodically. So just repeat this process of opening the jar, refreshing the air, blending for 15 seconds, and letting it stand for 2 or 3 minutes. It will most likely take about 10 or 12 minutes to completely oxidize this first portion, so start testing if after 3 or 4 cycles of doing this. OK, two more minutes have passed, and it's been about 10 minutes since we've added the first portion of ascorbic acid. [end of treansciipt]
Published on Jul 15, 2014
http://www.recverin.com Learn how to make do-it-yourself DHAA (dehydroascorbic acid), and see remarkable blood plasma absorption results comparing oral doses of common vitamin C tablets, liposomal vitamin C, and the oxidized form of vitamin C called DHAA. http://www.recverin.com/aboutus.sc;jsessionid=B7D82F3AB5CB03980D27F4F0F32485CB.p3plqscsfapp006Questions about the Naturally-Occurring Forms of Vitamin C
Most people recognize the name "Ascorbic Acid (AA)" and consider it synonymous with "Vitamin C." Actually, AA is one of the two naturally-occurring forms of Vitamin C. L-Dehydroascorbic Acid is the other. The L- designation refers to the biologically active stereoisomer of each of these compounds. Our products only contain the L- forms, and we frequently drop the L- from the names for simplicity.
One of the most misunderstood terms by people seeking Vitamin C skincare products is the word "oxidized." It is a term used in chemistry to describe a transfer of electrons. AA becomes DHAA by being oxidized. In most solutions, AA can easily be oxidized to DHAA. Unfortunately, in those solutions, DHAA very rapidly decomposes in a complex series of additional chemical reactions. Therefore many people have come to equate the "oxidation" of AA in a product with the "decay, destruction, or decomposition" of the Vitamin C. Every nutrient or other substance that is needed by a cell in your body must somehow get inside of that cell. Typically those substances are absorbed from the fluids that the cells are bathed in. But that doesn't mean these substances can just "soak" into the cell. The absorption of most substances is very highly controlled by means of specialized molecules or structures in the cell membrane. The absorption of Vitamin C is very highly controlled by means of special "transporters." The two naturally-occurring forms of Vitamin C use different types of transporters to be absorbed. Transporters for AA are found on some cell types, and transporters for DHAA are found on all cell types. AA is absorbed by its transporters at a relatively slow rate as compared to the absorption of DHAA by its transporters. DHAA is also absorbed to higher levels inside the cell. DHAA is converted almost instantly into AA once it gets inside the cell, so you see that both mechanisms are used to supply the cells with the same essential substance. In chemical terms, to be "unstable" means that a substance changes into a different substance relatively quickly. These changes are chemical reactions, and the rate of a chemical reaction is hugely affected by conditions such as heat, light and the presence of air. Therefore stability is completely relative to conditions. Both AA and DHAA are stable for years in dry, powdered form, stored in dark bottles with all the oxygen removed, and kept cool. AA is extremely stable when dissolved in polyols such as glycerin, and DHAA is reasonably stable also. But what if you dissolve them in water? Well, AA is unstable in this condition, and DHAA is even less stable. So the AA solution will lose half of its original AA in a matter of months, or even weeks (depends on what temperature you store it at, for one thing). Most skincare products that contain Vitamin C are water-based solutions in which both forms are unstable. Among Vitamin C researchers, DHAA is very well-known and is the subject of intense and on-going investigations. It was described in a 2000 scientific review as "an important, interesting but somewhat enigmatic compound in biological systems" with "many unique properties that set it apart from ascorbic acid." (ref 1) (1) Deutsch JC (2000) Dehydroascorbic acid. J Chromatogr A 881: 299-307
 Reactive Oxygen Species (ROS) are chemically-reactive molecules containing oxygen. They are also called "free radicals." The basic things to know in regard to your skin is that these ROS are formed in the skin in many ways, including normal oxygen metabolism, immune-system function, and exposure to environmental oxidants and UV light; and that ROS can damage the lipids, proteins, and DNA in your skin. Vitamin C has three very distinct functions related to skin collagen; protection, stimulation, and production.
Yes, topical Vitamin C can minimize the appearance of fine lines and wrinkles. It's hydrating properties combined with its collagen-stimulating and collagen-protecting properties probably explain how (see above). But it concerns us that people who don't immediately see this effect often conclude that Vitamin C doesn't work for them. Making wrinkles go away is quite different from preventing them from forming in the first place. The long-term effect of topical Vitamin C on your appearance is not something that can or should be measured on the basis of whether you look 10 years younger in just a few weeks. Vitamin C provides anti-oxidant protection to ALL of the lipids, DNA, and proteins in the skin, including collagen. We believe that topical Vitamin C should be a staple of your skincare regimen for the long-term health and youthful appearance of your skin.  Obviously, no chemical, and nothing you can buy in a bottle is going to stop you from being one day older tomorrow. Topical Vitamin C can reduce existing signs of aging such as wrinkles, and it will reduce future signs of aging by assuring that the most important anti-oxidant in the skin is not depleted. The aging effects of insufficient anti-oxidant protection are cumulative. The damage that your skin has suffered earlier in your life is revealed in the appearance of your skin today. Yet many people never start thinking about topical Vitamin C until they see the lines and wrinkles. Would you wait until you had tooth decay before you started brushing? Does Vitamin C protect the skin from Sun Exposure?  Vitamin C is not sunscreen...it doesn't block or absorb UVA or UVB radiation. But many studies have shown that the redness of sunburn, and the formation of what are known as "sunburn cells," are both reduced by the topical use of Vitamin C. This is believed to be the result of neutralizing ROS as discussed above. Everyone should use sunscreen. Any dermatologist will tell you that a person who intentionally exposes her/his skin to intense sun is foolish. But sunbathing and tanning salons remain very popular. Since it is well known that the Vitamin C level in skin can be drastically depleted by intense sun or UV exposure, we believe that suntanners are among those who critically need topical Vitamin C. About half of the Vitamin C in normal skin is in the oxidized form known as Dehydroascorbic Acid (DHAA) (refs 1 and 2). This is highly unusual. In all other body tissues that have been measured (including adrenals, pituitary, liver, spleen, lung, kidney, testes, thyroid, heart, muscle, brain, liver, white blood cells, pancreas, eye, and plasma) Vitamin C exists almost exclusively as ascorbic acid (AA), with very little DHAA (ref 6). It is unknown why Mother Nature tries to maintain unusually high levels of DHAA in the skin. (1) J Invest Dermatol 100:260-265 (1993)
(2) J Invest Dermatol 102:122-124 (1994) (3) J Invest Dermatol 96:590A (1991) (4) BiochemJ 345:665-672 (2000) (5) J Biol Chem 270(21):12584-12592(1995) (6) Ann NY Acad Sci 258(1) 103-118 (1975) What Makes DHAA Superior for Topical Use? DHAA has two chemical properties that make it superior to AA for topical use. Namely, DHAA is not ionized in solution, and it is more lipophilic than AA. These properties mean it is more gentle and can penetrate the stratum corneum more easily. (The stratum corneum is the outer layer of the skin comprised of dead, flattened skin cells. It creates a natural and desirable barrier for the protection of the living cells below, but also tends to prevent the absorption of topically applied substances, particularly ionized, water-soluble substances). In our own study, over 12 times as much DHAA was absorbed after 4 hours (see ref 1). DHAA also has three biological properties that make it superior. Namely, it is absorbed by cells much more quickly than AA, it can be absorbed by all cell types whereas AA can only be absorbed by some cell types, and thirdly it can be absorbed to higher levels in cells.
Because ReCverin 50/50™ is formulated with both AA and DHAA, it provides much more Vitamin C for the skin using lower, more gentle concentrations. Chemical derivatives are synthetic "Vitamin C" molecules that are made by chemically linking other molecules to AA molecules. These derivatives are generally more stable than natural Vitamin C in most skincare products. Manufacturers use these derivatives to improve the shelf-life of their products. This definitely benefits the manufacturer, but the question is, "Do AA derivatives benefit you?" The next two FAQs discuss this question. Some of the commonly used derivatives are named Ascorbyl Palmitate (AP), Magnesium Ascorbyl Phosphate (MAP), Sodium Ascorbyl Phosphate (SAP), and Tetra-Isopalmitoyl Ascorbic Acid (TETRA, also called Tetrahexyldecylascorbate). Are these physiologically useful forms of Vitamin C for topical use? They certainly aren't natural; not one of these compounds exists in nature. (1) J Invest Dermatol 119:1103-1108 (2002)
By Percent Concentration, how do derivatives compare to natural Vitamin C? It takes a lot more of the commonly used derivatives to equal the same molar concentration of natural Vitamin C. What Percent Concentration of each derivative is comparable to 10% Ascorbic Acid?
  Why does it take so much more of a derivative to be equal to Ascorbic Acid? Imagine having a pound of golf balls and a pound of ping-pong balls. There are about 11 golf balls per pound, but there are many more ping-pong balls in a pound because each ball weighs less. Now, if you assume each ball is equal in value, is it better to have a pound of golf balls or a pound of ping-pong balls? Here is a link to an excellent discussion named Molecular Weight and the Mole from Kimball's Biology Pages by Professor John W. Kimball. Our goal is to provide the purest natural Vitamin C serums in the world. Every ingredient in a skincare product, be it a product enhancer like perfume, be it an active ingredient such as Vitamin E, or be it a plant extract like Acai or Green Tea, adds another possible source of skin reaction or irritation. These additives can also affect the stability of Vitamin C. We want everyone to be able to use topical Vitamin C, with the confidence that they are using a pure, natural product.
Why is there so little DHAA in most people's diet?  Since DHAA is formed by oxidation of AA, there is a small amount in every food that has any significant amount of Vitamin C. But since DHAA is an extremely unstable chemical (far less stable than AA), very little can accumulate in foods, because it is destroyed at a more rapid rate than it is formed. However, there is one significant dietary source of DHAA. Many fresh raw vegetables, such as cabbage, squashes, pumpkins, peas, string beans, Lima beans, sweet corn, Swiss chard, carrots, parsnips, and spinach contain an enzyme called ascorbic acid oxidase that can rapidly convert AA into DHAA. AA is the predominant form of Vitamin C in these vegetables, but when the vegetables are crushed the enzyme can convert the AA into DHAA. This reaction is extremely fast--much of the AA is converted into DHAA right in your mouth while you are chewing the vegetable! Therefore in this instance, DHAA is formed at a rate much greater than it is destroyed, and the accumulated DHAA is swallowed and absorbed. (1) J Biol Chem 116(2):717-725 (1936)
ReCverin C™ is probably the most stable liquid formulation of L-Ascorbic Acid in the world. Our room temperature storage studies (20 degrees C., 68 degrees F.) show neglible deterioration over a one year period, with no visual yellowing, and retention of more than 99% reducing activity. Accelerated studies (meaning storage at elevated temperatures) suggest the stability may be much longer. We feel that we are being very conservative when we say that you can easily expect ReCverin C™ to retain more than 95% of its stated activity for at least a year. BibliographyReCverin LLC is founded in the science of Vitamin C, with particular focus on the oxidized form called DHAA. We believe that the more you know about this science, the more you will appreciate the value of our products. We believe the most reliable information can be found in scientific literature such as published research articles and patents. Therefore we dedicate this column of our website to a bibliography of titles, with links to the articles, that are pertinent to a general understanding of Vitamin C, with specific emphasis on DHAA. These titles are selected, for the most part, because the research contains experimental data that illustrates the chemical nature or biological behavior of Vitamin C. ReCverin LLC does not necessarily agree with the interpretations or conclusions drawn from that data by the authors. In particular, any statements about disease found in any of these articles are strictly the opinions of their respective authors. Our products are not intended to diagnose, treat, cure, or prevent any disease. (2016) Genetic Variation in Human Vitamin C Transporter Genes in Common Complex Diseases (2015) Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH (2015) Genetic Variants in GLUT14 Gene Enhance Susceptibility to Inflammatory Bowel Disease (2014) Vitamin C Deficiency – Part 3 (2014) The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism (2014) Subcellular compartmentation of ascorbate and its variation in disease states (2014) Role of GLUT1 in regulation of reactive oxygen species (2013) Regulation of Vitamin C Homeostasis during Deficiency (2011) High dietary fat and cholesterol exacerbates chronic vitamin C deficiency in guinea pigs (2011) Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury (2010) Glucose transporter 10 and arterial tortuosity syndrome: The vitamin C connection (2009) Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2 (2008) Antiviral effects of ascorbic and dehydroascorbic acids in vitro (2007) Vitamin C: Biosynthesis, recycling and degradation in mammals (2004) Vitamin C Is a Kinase Inhibitor: Dehydroascorbic Acid Inhibits IκBα Kinase β (2003) Recycling of Vitamin C by a Bystander Effect (2002) Vitamin C Prevents DNA Mutation Induced by Oxidative Stress (2000) Ascorbate oxidation is a prerequisite for its transport into rat liver microsomal vesicles (1998) Absorption, transport, and disposition of ascorbic acid in humans (1997) Glucose Transporter Isoforms GLUT1 and GLUT3 Transport Dehydroascorbic Acid (1996) Gluconeogenesis from ascorbic acid: ascorbate recycling in isolated murine hepatocytes (1994) Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin (1993) Ascorbic acid recycling in human neutrophils (1956) Aging: A Theory Based on Free Radical and Radiation Chemistry--by Denham Harman (1944) Water Soluble Vitamins in Sweat (1937) The Oxidation of Ascorbic Acid and its Reduction In Vitro and In Vivo (1936) Vitamin C in Vegetables: Ascorbic Acid Oxidase (1934) The urinary excretion of ascorbic and dehydroascorbic acids in man
|
|||||||
|
|
 It's simply wonderful to have at our fingertips the ability to communicate with the entire world - from the comfort of our home - and be given the opportunity to present the most helpful and valuable information imaginable. And so it was that on the eve of July 4th, Independence Day in America, I should happen to stumble upon a video titled "Liposomal vs. Oxidized Vitamin C and DIY DHAA: The Amazing Green Smoothie" from
It's simply wonderful to have at our fingertips the ability to communicate with the entire world - from the comfort of our home - and be given the opportunity to present the most helpful and valuable information imaginable. And so it was that on the eve of July 4th, Independence Day in America, I should happen to stumble upon a video titled "Liposomal vs. Oxidized Vitamin C and DIY DHAA: The Amazing Green Smoothie" from 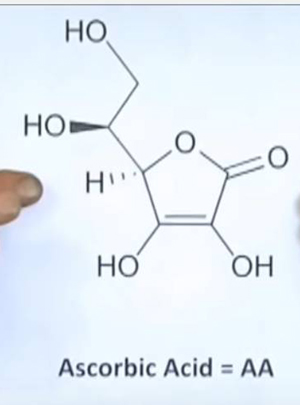
 Linus Pauling recommended taking the ascorbate form of Vit. C (a mineral combined with ascorbic acid, such as Sodium Ascorbate or Calcium Ascorbate) because the ascorbate form has a pH of about 7 and it won't upset your stomach if you take a very large amount at one time (such as a level teaspoon which is equivalent to 5 grams). If I got really ill or came down with a bad systemic infection, I would take 5 grams of ascorbate every 4 hours and within 24 hours, I usually felt 1000% better and well on my way to complete recovery
Linus Pauling recommended taking the ascorbate form of Vit. C (a mineral combined with ascorbic acid, such as Sodium Ascorbate or Calcium Ascorbate) because the ascorbate form has a pH of about 7 and it won't upset your stomach if you take a very large amount at one time (such as a level teaspoon which is equivalent to 5 grams). If I got really ill or came down with a bad systemic infection, I would take 5 grams of ascorbate every 4 hours and within 24 hours, I usually felt 1000% better and well on my way to complete recovery 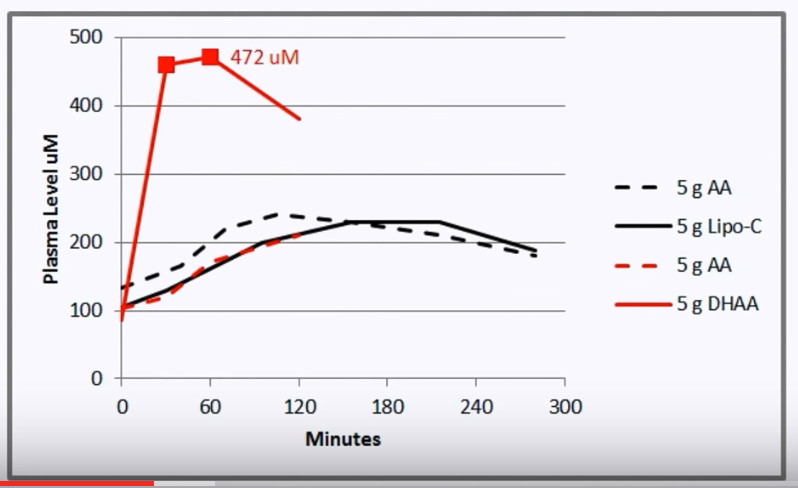 To demonstrate the greater blood plasma uptake of DHAA, Doug ran his own experiment by consuming 5 grams of 1) ascorbic acid (AA) on Day 1 (shown in dashed red), and 5 grams of DHAA (about 1/3 of the green smoothie) on Day 2 and measured the Vit. C blood plasma levels at 60 minute intervals out to 5 hours.
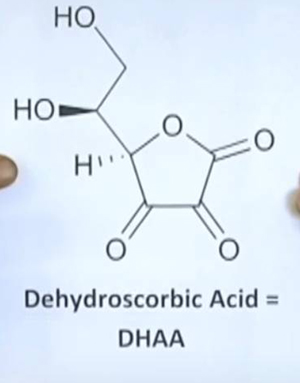
To demonstrate the greater blood plasma uptake of DHAA, Doug ran his own experiment by consuming 5 grams of 1) ascorbic acid (AA) on Day 1 (shown in dashed red), and 5 grams of DHAA (about 1/3 of the green smoothie) on Day 2 and measured the Vit. C blood plasma levels at 60 minute intervals out to 5 hours.  Dehydroascorbic Acid (DHAA) is the oxidized form of Vitamin C; it is one of the two naturally-occurring forms. The oxidation of Ascorbic Acid (AA) to DHAA explains the powerful anti-oxidant activity of AA. DHAA can be converted back into AA by the body, so Vitamin C is "recycled." Unfortunately though, the molecules themselves eventually break down, so the body requires a continuous supply of more Vitamin C.
Dehydroascorbic Acid (DHAA) is the oxidized form of Vitamin C; it is one of the two naturally-occurring forms. The oxidation of Ascorbic Acid (AA) to DHAA explains the powerful anti-oxidant activity of AA. DHAA can be converted back into AA by the body, so Vitamin C is "recycled." Unfortunately though, the molecules themselves eventually break down, so the body requires a continuous supply of more Vitamin C.